JCRB1846 EEB
Cell information
Cell type:general cells (View Pricing Information)
| JCRB No. | JCRB1846 | Cell Name | EEB |
|---|---|---|---|
| Profile | Human cell line derived from erythroblastic leukemia. | Other Name | |
| Animal | human | Strain | |
| Genus | Homo | Species | sapiens |
| Sex | M | Age | 49-year-old |
| Identity | not done | Tissue for Primary Cancer | |
| Case history | early erythroblastic leukemia | Metastasis | |
| Tissue Metastasized | Genetics | ||
| Life Span | infinite | Crisis PDL | |
| Morphology | lymphocyte-like | Character | |
| Classify | tumor | Established by | Muroi K. |
| Registered by | Kazuo Muroi | Regulation for Distribution | |
| Comment | In publishing research results obtained by the use of the BIOLOGICAL RESOURCE, a citation of the literature ref.(Leuk Res 2006;30:829-39) designated by the DEPOSITOR is required. | Year | |
| Medium | StemSpan SFEM + 15% FBS | Methods for Passages | dilution ( 1 : 4-6 split ) |
| Cell Number on Passage | Race | ||
| CO2 Conc. | 5% | Tissue Sampling | |
| Tissue Type |
| Detection of virus genome fragment by Real-time PCR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected DNA Virus | tested | Detected RNA Virus | tested | ||||||
| CMV | - | parvoB19 | - | HCV | - | HTLV-1 | - | ||
| EBV | - | HBV | - | HIV-1 | - | HTLV-2 | - | ||
| HHV6 | - | HTLV-1 | - | HIV-2 | - | HAV | - | ||
| HHV7 | - | HTLV-2 | - |
-/negative. +/positive. nt/not tested. (positive (+) does not immediately mean the production of infectious viral particles.) |
|||||
| BKV | - | HIV-1 | - | ||||||
| JCV | - | HIV-2 | - | ||||||
| ADV | - | HPV18 | - | ||||||
| Notes | |||||||||
| Reference | |
|---|---|
| Pubmed id:16332389 | Establishment and characterization of a new erythroblastic leukemia cell line, EEB: phosphatidylglucoside-mediated erythroid differentiation and apoptosis. Kawano-Yamamoto C,Muroi K,Nagatsuka Y,Higuchi M,Kikuchi S,Nagai T,Hakomori SI,Ozawa K Leuk Res. 2006 Jul;30(7):829-39 |
| Images |
|---|
  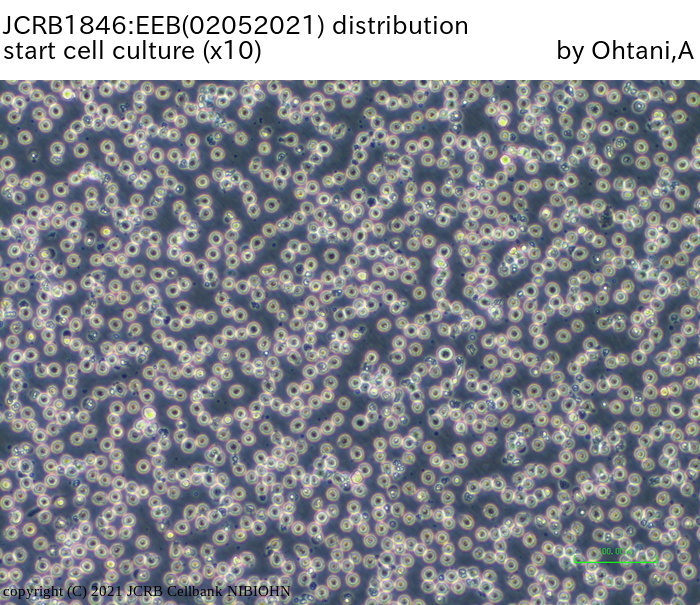  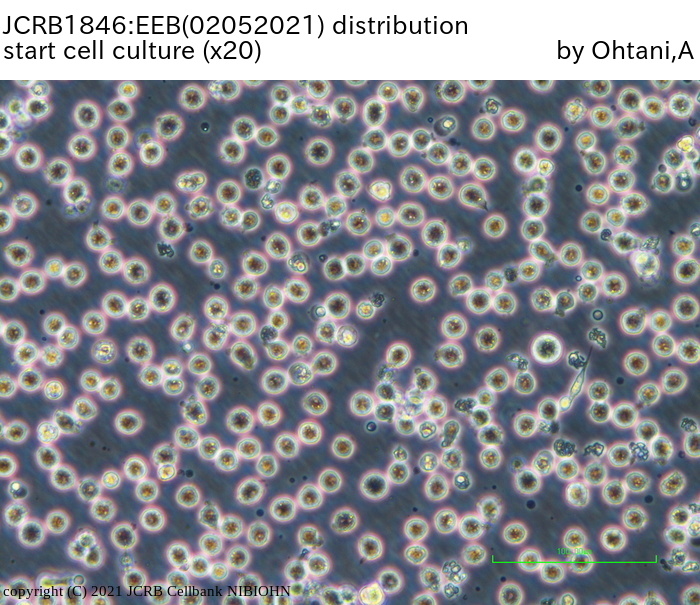 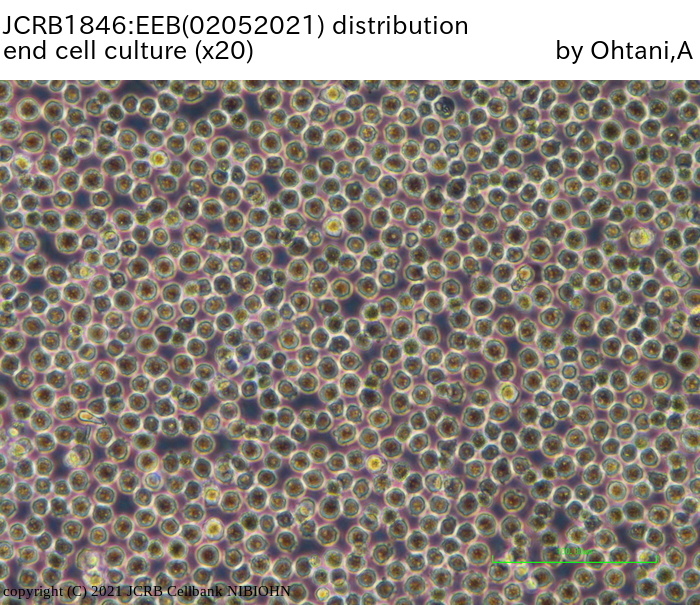 |
LOT Information
Viability/Growth rate/Cell number are represented as actual values measured at lot presentation in JCRB, but are not guaranteed values. Additionally, the doubling time is a rough value measured during passages.| Cell No. | JCRB1846 | Cell Name | EEB |
|---|---|---|---|
| LOT No. | 02052021 | Lot Specification | distribution |
| Medium | StemSpan SFEM(VERITAS Cat No.ST-09650) with 15% heat inactivated fetal bovine serum(SIGMA,Cat#172012,Lot#12J396) | Temperature | 37 C |
| Cell Density at Seeding | 1.04-2.29x10^5 cells/ml | Methods for Passages | dilution |
| Doubling Time | NT | Cell Number in Vial (cells/1ml) | 5.01x10^6 |
| Viability at cell freezing (%) | 97 | Antibiotics Used | free |
| Passage Number | p4* | PDL | NT |
| Sterility: MYCOPLASMA | - | Sterility: BACTERIA | - |
| Sterility: FUNGI | - | Isozyme Analysis | NT |
| Chromosome Mode | NT | Chromosome Information | NT |
| Surface Antigen | NT | DNA Profile (STR) | D5S818:11 D13S317:9,12 D7S820:10 D16S539:10,12 VWA:17 TH01:7 AM:X,Y TPOX:8,9 CSF1PO:11 |
| Adhesion | No | Exoteric Gene | NT |
| Medium for Freezing | BAMBANKER(LYMPHOTEC Inc.,CS-02-001,NIPPON Genetics Co., LTD) | CO2 Conc. | 5% |
| Viability immediately after thawing (%) | 95 | Additional information |
| Images |
|---|
      |